
Dr Saadia Aslam, Clinical Research Fellow, NIHR Leicester Biomedical Research Centre and Mr Michael Walters, Senior Trial Manager, Leicester Clinical Trials Unit share opportunities for BSE members to get involved in the EASY-AS research project.
The EASY-AS project is British Heart Foundation (BHF) funded research with a clinical trial sponsored by the University of Leicester and run by the team at Leicester Clinical Trials Unit. Not only could your role be essential in making this trial a success but you would also be provided with the opportunity to develop yourself in a research environment with a view to becoming a future Principal Investigator (PI) by participating in the NIHR Associate PI scheme.
The EASY-AS trial
The EASY-AS trial is a BHF funded Randomised Controlled Trial of Early valve replacement in severe ASYmptomatic Aortic Stenosis.
Aortic Stenosis (AS) is the most common valve disease requiring intervention but the risks and benefits of a strategy of early aortic valve replacement (AVR) versus expectant management in asymptomatic patients are unclear. The timing of the intervention in patients with severe, but asymptomatic AS is controversial. Two small randomised trials (RECOVERY [NCT01161732]and AVATAR [NCT02436655]) have suggested that there is a benefit in selected populations but larger pragmatic trials are needed to provide definite proof.
This is where EASY AS comes in. Using a pragmatic international, prospective parallel group randomised controlled trial (RCT) design, up to 2844 patients with severe asymptomatic AS will be randomised 1:1 to either early AVR (surgical or transcatheter valve implantation [TAVI]) or watchful waiting. The trial successfully completed its Vanguard Phase in July 2022, recruiting 180 patients across 26 UK sites. As part of the Main Phase, we are aiming to open a further 80 to 100 sites across the UK. Overall recruitment is 373 and the target is to recruit an additional 2471 participants over the next 4 years from across UK, Europe, Australia, New Zealand and Asia.
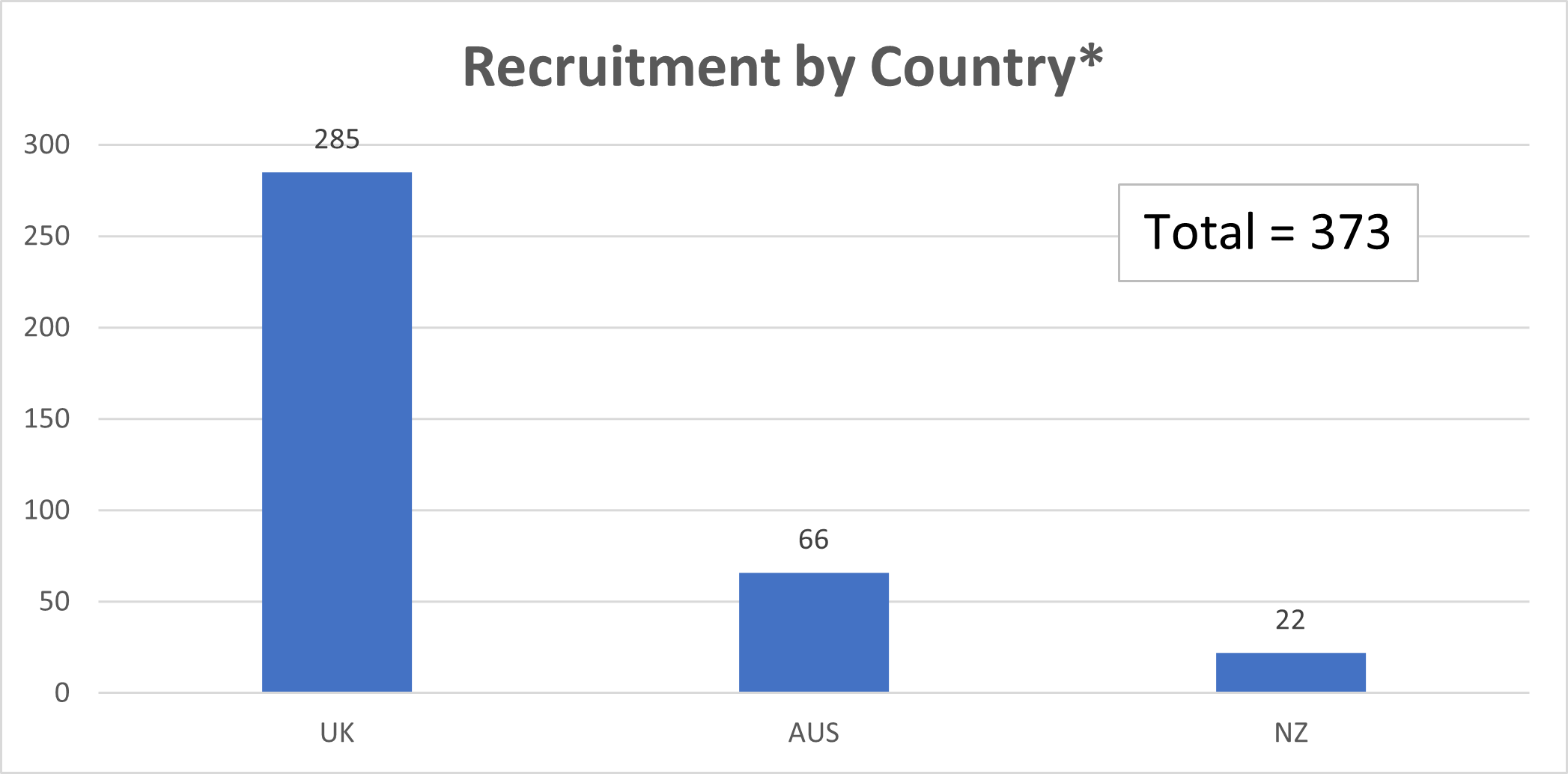
*The graph above shows current recruitment correct as of 11/05/2023
EASY-AS will be the largest, pragmatic trial to date in this area and will inform international guidelines and provide strong evidence regarding the management of severe asymptomatic aortic stenosis. We would very much welcome your participation in this exciting study.
The Role of the Cardiac Physiologist in EASY-AS
Cardiac physiologists are often the first secondary care professionals to identify and have contact with patients with severe aortic stenosis. This initial interaction with these patients could help the EASY-AS trial.
The successful identification of patients with severe aortic stenosis will allow clinicians at participating sites to approach these patients to discuss their treatment options and potential involvement in EASY AS whilst they remain asymptomatic.
The EASY-AS trial is also participating in the NIHR Associate PI Scheme. This is a six month in-work training opportunity for any healthcare professional who wants to develop their research skills and potentially undertake future research as a PI, and formal recognition of your involvement in the trial.
Examples of research activities:
- raising the profile of the study within the echo department
- engagement with echo/clinical team and dissemination of trial information
- highlighting and recruiting suitable patients based on echo criteria to other members of the research team
- engaging with the NIHR Associate Principal Investigator Scheme
- involvement in research visits
If you would be interested in becoming involved in this trial but your site is not currently participating then please discuss this with one of your cardiologists who has an interest in valve disease and/or imaging. Please contact the trial centre team at [email protected] for any queries or further information. You can also follow us on Twitter: @easyas_trial
Participating sites
|
Aberdeen RI
|
Cumberland
|
Lewisham
|
Queen Alexandra
|
James Cook UH
|
|
Aintree UH
|
Derriford Hospital
|
Lincoln CH
|
Queen Eliz, B’ham
|
Torbay Hospital
|
|
Airedale NHSFT
|
Doncaster RI
|
Liverpool H&C
|
Royal Devon & Exe
|
Wansbeck GH
|
|
Basildon UH
|
Glenfield
|
Maidstone & TW
|
RI Edinburgh
|
Warwick Hospital
|
|
Basingstoke & NH
|
Huddersfield RI
|
Norfolk & Norwich
|
Royal Sussex CH
|
Wythenshawe
|
|
Blackpool Victoria
|
Imperial College
|
North Manchester
|
Southampton GH
|
Yeovil DH
|
|
Castle Hill
|
Kettering GH
|
North Tees & Hart
|
St Barts, London
|
Royal Liverpool
|
|
County Durham
|
Leeds GI
|
Poole Hospital
|
St Thomas London
|
Swansea Bay
|
|
Sandwell GH
|
Walsall Hospital
|
Russels Hall
|
Western HSC
|
North Linc & Goole
|
|
George Eliot
|
Raigmore
|
Dorset CH
|
Royal Cornwall
|
Somerset
|
|
Ayr & Arran
|
South Eastern HSC
|
Freeman Hospital
|
|
|